Written by Matt Jalink – MSc Candidate in Community Health and Epidemiology
“The world is running out of antibiotics” – WHO report
Antimicrobial resistance (AMR) occurs when bacteria, viruses and other microorganisms change in ways that cause existing medications (ex. antibiotics – bacterial infections, antivirals – viral infections) to be ineffective. These resistant microorganisms are commonly termed “superbugs” and are a major public health concern due to their ability to spread in populations and impose large individual and society costs (WHO, 2017). AMR organisms cause more than 2 million infections and approximately 23,000 deaths each year in the United States (Marston, 2016). The economic costs are also substantial, estimated at $20 billion in excess medical spending each year (Marston, 2016).
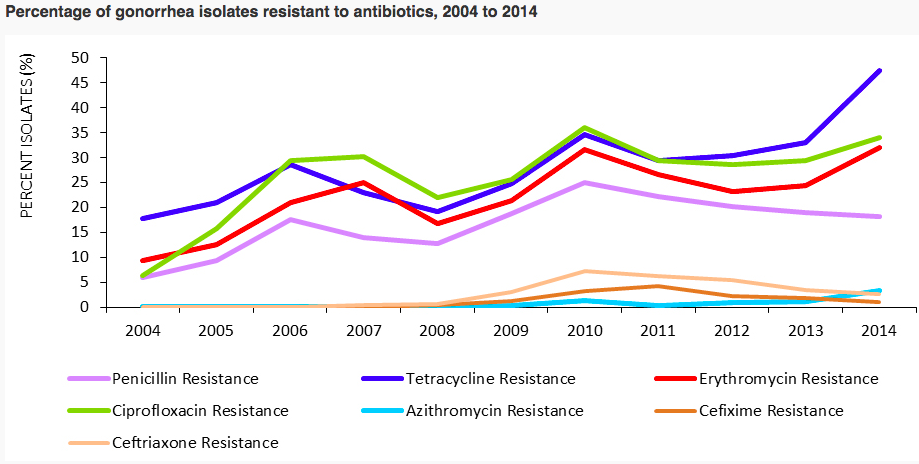
AMR is causing traditionally curable diseases are becoming increasingly more difficult to treat. Data collected from 77 countries shows that antibiotic resistance in gonorrhea is causing infections to be harder and sometimes impossible to treat, and is on the rise (WHO, 2017).
(WHO, 2017)
Factors Influencing Antimicrobial Resistance
Microorganisms resistant to modern medicine have existed for thousands of years, and appear to predate the antibiotic era. Frozen samples collected from Yukon contained bacteria with resistant mutations 30,000 years before the discovery of penicillin, the world’s first antibiotic (D’Costa, 2011). But despite naturally occurring antibiotic resistance, the number of cases of resistant microorganisms continues to rise, which can be attributable to human activity.
One example is through the overuse of antibiotics. The overuse of antibiotics is in many cases unwarranted, and regiments are frequently not completed, leading to drug resistance. A number of drug-resistant infections are found in hospitals, such as Methicillin-resistant staphylococcus aureus (MRSA). However, impatient antibiotic usage represents only 38.5% of the total antibiotic market (Marston, 2016). A recent American study found that 12.6% of outpatient visits in the US resulted in the prescription of an antibiotic, and 30% of those prescriptions were deemed inappropriate (Marston, 2016). Canada ranks in the middle of 30 European countries in outpatient antimicrobial use.
(Canada, 2017)
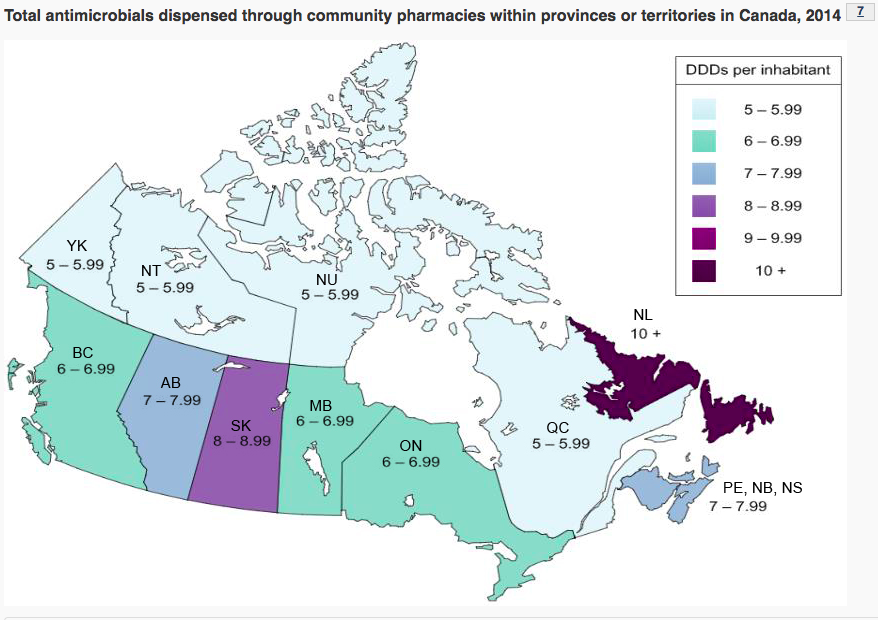
The overuse of antibiotics largely stems from over-prescribing of medications for conditions that don’t require antibiotics for treatment. The level of antibiotic prescription across Canada varies by province, with the highest levels found Newfoundland and Labrador.
(Canada, 2017)
Outside of North America and Europe, outpatient antibiotic prescription can account for 20 – 100% of antibiotic use depending on location (Morgan, 2011). In many of these nations, antibiotics are dispensed directly to the consumer, and are more likely to be inappropriately selected and taken at doses below optimum levels (Morgan, 2011).
With increasing levels of drug resistant infections, new medications are needed. However, the pace of new antibiotic development has slowed considerably. There were 16 antibiotics were approved by the FDA between the years 1983 and 1987, but only 2 new antimicrobial drugs were approved between 2008-2012 (Boucher, 2013) and 5 in total since 2012 (Marston, 2016). There are certain characteristics of the antibiotic market that likely hinder pharmaceutical industry investment in new drug development. The limited duration of treatment, relative low prices per dose, and the potential for the rapid emergence of resistance (uncertain market longevity) all diminish revenue prospects for new antimicrobial drugs (Marston, 2016).
The agricultural sector also contributes towards the growing resistance of microorganisms. An association has been found between antibiotic consumption by animals and the existence in humans of microorganisms resistant to the same antibiotic classes (Chantziaras, 2014). In 2014, approximately 82% of antimicrobials important to human medicine were distributed and sold for use in food-producing animals (Health Canada, 2014). The influence of the agricultural use of antibiotics on human health has drawn a range of responses, including policies in Europe banning antibiotic use for animal growth, and regulation from the Food and Drug Administration (FDA) encourage similar practices.
Response, methods to combat antimicrobial resistance
Surveillance of antimicrobial resistance starting from local global health departments is very important for managing and controlling AMR. Surveillance captures information about AMR incidence, prevalence, and trends from particular regions. This information can be pooled together to see national and global trends of AMR. The Global Antimicrobial Surveillance System (GLASS) shares data internationally on antimicrobial resistance to inform decision-making, drive local and regional action, and provide an evidence base for action and advocacy (WHO, 2017). In Canada, AMR is monitored by the Canadian Antimicrobial Resistance Surveillance System (CARSS).
(WHO, 2017)
Basic public health measures like sanitation, hand washing, and food and water security can decrease the spread of AMR (WHO). Public Education Campaigns have proven effective on reducing inappropriate prescribing of antibiotics in Belgium and France (Gelband, 2015). There has also been a substantial effort to address inappropriate antibiotic use within hospitals; including implementation of antimicrobial stewardship programs, which seek to reduce inappropriate antibiotic and antimicrobial selection and reduce the inappropriate use of broad-spectrum antimicrobials. However, these stewardships have had limited implementation and acceptance in hospitals (Marston, 2016). Vaccines have a role as well. It is estimated that improved vaccine covered for Streptococcus pneumonia could prevent 11.4 antibiotic days per year in children under 5 years of age worldwide (Laxminarayan, 2016).
Citations
Canada, P. (2017). Government of Canada’s response to antimicrobial resistance – Canada.ca. [online] Canada.ca. Available at: https://www.canada.ca/en/public-health/services/antibiotic-antimicrobial-resistance/government-canada-response-antimicrobial-resistance.html [Accessed 13 Oct. 2017].
Chantziaras I, Dewulf J, Boyen F, Callens B, Butaye P. Antimicrobial resistance prevalence of pathogenic and commensal Escherichia coli in food-producing animals in Belgium. Vlaams Diergeneeskundig Tijdschrift. 2014;83(5):225-33.
D’costa VM, King CE, Kalan L, Morar M, Sung WW, Schwarz C, Froese D, Zazula G, Calmels F, Debruyne R, Golding GB. Antibiotic resistance is ancient. Nature. 2011 Sep 22;477(7365):457.
Gelband H, Molly Miller P, Pant S, Gandra S, Levinson J, Barter D, White A, Laxminarayan R. The state of the world’s antibiotics 2015. Wound Healing Southern Africa. 2015 Jan 1;8(2):30-4.
Laxminarayan R, Matsoso P, Pant S, Brower C, Røttingen JA, Klugman K, Davies S. Access to effective antimicrobials: a worldwide challenge. The Lancet. 2016 Jan 15;387(10014):168-75.
Marston HD, Dixon DM, Knisely JM, Palmore TN, Fauci AS. Antimicrobial resistance. Jama. 2016 Sep 20;316(11):1193-204.
Morgan DJ, Okeke IN, Laxminarayan R, Perencevich EN, Weisenberg S. Non-prescription antimicrobial use worldwide: a systematic review. The Lancet infectious diseases. 2011 Sep 30;11(9):692-701.
World Health Organization (WHO). Antimicrobial Resistance. 2017.


Leave a Reply