
Image adapted from: Johnson & Foster, 2028, Nature Reviews Microbiology
Before the emergence of multicellular life forms, energy-dense nutrients were picked up by the simplest microorganism directly through the cell membrane, but these organisms were limited in the repertoire of small nutrients they could digest. With time, multicellular clumps formed allowing the individual cells to conserve energy by specializing in metabolism of specific kinds of nutrients so that labor was distributed. The intricate mechanisms of communication between cells in a group and further differentiation of roles eventually allowed the inner cells to mostly focus on food digestions, while outer cells layers played protective and movement roles. Gradually, the inner cell layer evolved into the gastrointestinal (GI) tract, while other organs evolved into supporting organs. Hand in hand with the process of cell layer differentiation, external microorganisms, unrelated to the animal kingdom, (i.e. bacteria) learned to colonize the gut to take advantage of the nutrient exchange existing between the gut and the environment, by feeding on the undigested wastes and clearing debris. Bacterial microflora became an essential part of any mammalian GI tract.
After almost several centuries of neurosciences and microbiology existing as unrelated domains, the gut-brain has now emerged as a key system enabling the communication between bacteria and the brain. The communication is so tight and intricate that some may even claim the mammalian organisms have been manipulated by the host bacteria, akin to a puppet, to evolve into the complex, energy-expensive beings that forage for food for bacteria. Perhaps the interaction between the host and bacteria is not as one-directional as we rely on the bacteria for carrying out many of the biological functions. In fact, the perturbations of the bacterial composition in the gut can result in disease. Human research has increasingly highlighted a strong gut-brain connection. In one study conducted in Belgium and the Netherlands in more than 1,000 participants, researchers discovered that two specific strains of gut bacteria were consistently less prevalent in individuals suffering from depression. Another study found that people who consumed yogurt rich in probiotics experienced reduced brain activity in regions tied to stress, leading to a more relaxed and composed state. Gut-brain axis inter-talk is implicated in many other neurological disorders. Experimental and clinical studies suggest that gut microbiota significantly influence stroke risk and outcomes. During the acute phase of stroke, changes in gut microbial composition correlate with the severity of brain lesions. Singh et al. found that severe stroke in mice reduced gut bacterial diversity, with Firmicutes decreasing and Bacteroidetes increasing, which impaired gut motility and increased intestinal permeability. Variations in specific bacterial populations were reported across studies due to differences in experimental designs. Antibiotic-induced gut microbiome shifts prior to stroke improved outcomes, with reduced infarct size and better motor and sensory recovery. Notably, fecal transplants from young to aged mice improved stroke outcomes, highlighting age-related microbiome changes as a factor.
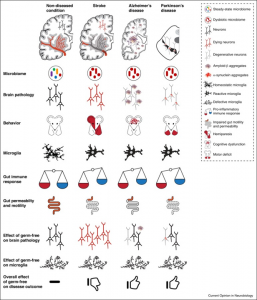
Adapted from: Benakis et al., 2020, Current Opinion in Neurobiology
Our knowledge of its specific impact on brain function, particularly in disease contexts, remains incomplete. Current research primarily highlights associations between gut microbiome composition and disease symptoms, but a deeper exploration into the mechanistic pathways of the interactions between brain and gut axis are needed.










